Abstract

Amongst the hematological malignancies, T-cell acute lymphoblastic leukemia (T-ALL/T-LL) constitute a class of highly aggressive cancers in children, adolescent, and young adult, marked by aggressive behavior and poor clinical response, notably for refractory and relapsing (R/R) cases. As seen in numerous cancers, genetic lesions leading to aberrant PI3K signaling (PI3KS) are frequent in T-ALL and convey adverse outcomes from limited therapy response to early relapse and dismal survival rates. We provide the largest comprehensive analysis of PI3KS alterations (PI3KSALT) in adult and pediatric T-ALL. PI3KSALT patients had a poor response to corticoids, and a higher relapse risk (77% vs 32%, p < 0.001). Despite similar complete remission rates, patients with PI3KSALT had shorter overall survival (5y-OS: 58% vs 74%, p = 0.007) and event-free survival (5y-EFS: 49% vs 65%, p = 0.008) and an increased cumulative incidence of relapse (5y-CIR: 39% vs 27%, p= 0.01). We report that PI3KSALT define a subclass of aggressive T-ALL with a poor prognosis, urging innovative therapies for these patients.
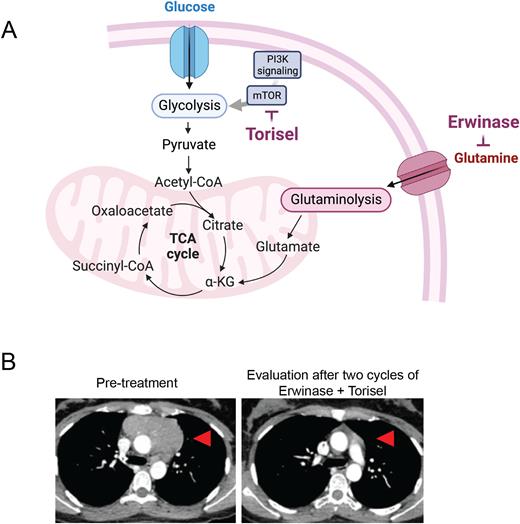
Primary samples and patient-derived xenografts (PDX) of PI3KSALT leukemia present an hyperglycolytic profile. PI3KSALT T-ALL cell lines mimic this addiction with a marked reliance on glucose. Surprisingly, while cell lines cannot survive glucose limitation, PI3KSALT PDX tolerate this starvation and maintain their energy levels, underpinning their ability to rewire their metabolic fluxes to adapt to a nutrient-deprived microenvironment. We showed that PI3KSALT PDX have unique metabolic plasticity upon starvation. These blasts use glutaminolysis to cope with glucose limitation and sustain the TCA cycle, while wild-type PI3KS fail to do so. Pharmaceutical inhibition of the PI3KS-mTOR pathway and glutamine metabolism under starvation validated this metabolic rewiring and presented marked cytotoxicity on blasts ex vivo.
We proposed a therapeutic strategy for PI3KSALT T-ALL based on the targeting of mTOR and glutamine. Erwinase, an L-asparaginase with glutaminase activity, efficiently synergizes with Torisel and demonstrates tumor clearance and prolonged survival in vivo in models of PI3KSALT T-ALL. Critically, we report the case of five patients suffering from R/R PI3KSALT leukemia treated with the association Erwinase/Torisel (ET). The two patients with T-ALL suffered from relapses following allogeneic stem cell transplantation (ASCT), while the three remaining patients with T-LL received up to three different lines of polychemotherapy before ET treatment. Four out of five patients experienced expected and acceptable side effects related to the ET combination. However, the fifth patient experienced significant and severe toxicity that led to the discontinuation of erwinase injection. All five patients achieved a response within one month after one or two courses. Patients suffering from R/R T-ALL achieved negative minimal residual disease, and patients with R/R T-LL presented a significant decrease in the mediastinal mass. The two patients suffering from relapsed T-ALL achieved negative minimal residual disease and patients with R/R T-LL experienced a significant decrease of the mediastinal mass based on early volumetric and/or metabolic evaluation assessment. After achieving complete remission, three patients received consolidation therapy consisting of allogeneic SCT in two patients, while the previously allografted third patient received donor lymphocyte infusion. Two patients remain alive as of today, while two patients rapidly progressed after the discontinuation of ET treatment and ultimately died from disease progression. One patient who received ASCT after achieving CR post-ET therapy eventually died from allograft-related toxicity.
R/R T-ALL/LL is associated with dismal prognosis and outcome, in part due to chemoresistance acquisition and the scarcity of the therapeutic armamentarium currently available for T-ALL/LL. Here, we unveiled a novel and promising treatment combining an asparagine and glutamine degrader (Erwinase) with a PI3KS inhibitor (Torisel) that should be considered as a therapeutic option in a bridge-to-transplant approach for R/R T-ALL/LL harboring PI3KS deregulation. We show that metabolic plasticity conveys a unique and targetable vulnerability in PI3KS-driven leukemia that show promising results in pre-clinical and clinical settings.
Disclosures
Boissel:GILEAD: Honoraria; AMGEN: Honoraria; ARIAD/INCYTE: Honoraria; ASTELLAS: Honoraria; NOVARTIS: Honoraria; SERVIER: Honoraria. Rousselot:Incyte: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Hermine:Kite/Gilead: Honoraria; Novartis: Research Funding; BMS: Honoraria, Research Funding; Inatherys: Research Funding; AB Science: Current equity holder in private company, Honoraria, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.